Healthcare Spending
As mentioned above healthcare costs continue to rise at a significant rate2. The Center for Medicare and Medicaid Services (CMS) projects that healthcare spending with rise 5.5% annually over the next decade reaching close to $6 trillion by 202710. This rate significantly exceeds both the recent nominal gross domestic product (GDP) and nominal wage growth rates11-12. Approximately 10% of total healthcare expenditures originates from pharmaceuticals in the United States, with the average American spending over $1,000 on their prescriptions each year5. Cost of prescription medications is cited as a major reason for patient nonadherence to medications with as many as 14% of those skipping prescriptions attributing their actions to the high cost. That figure rises to 24% when asking low-income Americans why they skip prescriptions and as high as 33% for uninsured Americans5. Nonadherence to prescribed medications is thought to cause about 125,000 deaths in the United States each year13. According to Viswanathan et al, nonadherence is thought to cost the American healthcare system between 100 and 289 billion dollars annually13. High prices of pharmaceuticals, leading to nonadherence and eventually much higher medical costs later due to this lack of preventative care creates somewhat of a positive feedback cycle costing the system, and thus consumers, more and more money. Much of the cost of pharmaceuticals is due to the large sums pharmaceutical companies are willing to put into research and development for new drugs. It’s estimated that the average price for the development of each new drug costs pharmaceutical companies about 800 million dollars14. Fully automating new drug development may be far off but there are still ways we can save money and reduce costs to consumers.
While the United States and other developed countries of the west struggle with high pharmaceutical prices, the problem with access to adequate and affordable healthcare persists in the rest of the world as well. As of 2001, some less developed countries, like Liberia for instance, had an annual per capita healthcare expenditure of only about 1 U.S. dollar15. Not only do the residents of less developed countries not have the funds for proper healthcare, they do not have the access for it and in many ways are less fortunate than even the low-income residents of more developed countries. For years the United Nations (UN) has been dedicated to the development of less fortunate countries, publishing their Millennium Development Goals (MDGs) in an effort to reduce poverty and to improve medical care and general quality of life16. Although, often forgotten, the purpose of science is to improve our quality of life. For these developing countries and for the low-income residents of more fortunate countries, it is essential that the pharmaceutical and healthcare industries achieve a higher efficiency in order to allow for greater affordability and, therefore, access. Through the development of new technologies and improving the efficiency of production methods, we increase the quality of care and purchasing power of the dollar respectively. With increased purchasing power, we increase the affordability of proper medical care and other life essentials. While it is the general trend in most industries that as technologies improve the cost to consumers decreases, the cost of pharmaceuticals remains out of reach for many, especially citizens of developing countries and those of lower income levels in developed countries. Consequently, it should be a goal of biotechnologists and pharmaceutical scientists to increase the efficiency of production in order to lower the cost to consumers.
Rising costs is not necessarily the norm across industries. In his book “The Cost Disease,” William Baumol discusses how the prices of other technologies get cheaper while medical care and pharmaceuticals continue to get more expensive17. Baumol argues that many industries have become largely automated and thus are more efficient resulting in lower prices. Industries such as healthcare and education, however, remain labor-intensive, which is one reason their prices remain high. The questions remains, what can we do to change this trend and lower costs? Although the focus of this paper will be to discuss scientific measures to reduce costs of prescription medications it is important to note that the most effective way to lower healthcare costs at least in the short term may indeed be through changes in political policy. From a scientific perspective, however, there are many avenues of research that may lead to more affordable pharmaceuticals. Improving the thermostability of drugs and vaccines, repurposing existing drugs for new applications, and developing cheaper or more efficient cell culture media used for drug production are all viable routes to lower costs to consumers.
Competition and Generics
Competition in a market is one of the best way to drive down prices. Unfortunately patents prevent competition for a new medication for as many as 20 years in the drug industry. In some circumstances, companies can effectively extend their monopoly on a recently off-patent drug by arranging “pay to delay” agreements with competitor companies. Such agreements involve paying rival companies to hold off on developing a generic version of the name-brand drug for a specified amount of time so as to maintain full market share for said drug.
Once generics begin to be produced they save consumers and governments a great deal of money. The Association for Accessible Medicines estimated that generic drugs lowered pharmaceutical spending by $253 billion in the U.S. in 2016, with $2.9 billion saved in Connecticut18. The cost difference is quite significant with generic drugs priced about 85% less than their brand-name counterparts.19 To demonstrate this cost difference another way, generics have 84% market share of U.S. pharmaceutical industry by volume but only 28% by value20. Interestingly, generics seem to be grasping more of the market share both in value and volume over the last 13 years in several EU countries at least, perhaps as more popular drugs come off patent20. New consumer facing software can also help lower costs on pharmaceuticals by exposing individual consumers to competitive or alternative products with a lower price. Companies like Rx Savings Solutions notify users of any more competitively priced generic products as well as prompt them to talk with their doctors about alternative medications for their condition that may decrease costs. A more expensive class of drugs, called biologics also have their own type of ¨generic” to increase competition in the market.
Biologics and Biosimilars
Biologics, also known as biopharmaceuticals, are a class of drugs produced from living organisms Biologics are typically large macromolecules with tens of thousands of atoms making them much larger than typical pharmaceuticals like aspirin, which has 21 atoms for instance (Figure 1). Administration of biologics are often via infusion or injection, and largely because of the size of the molecules, biologics are typically much less stable than typical pharmaceuticals. Most of their applications are in complex disease states such as cancer and autoimmune disorders. One of the most important pieces of information about biologics is their price tag. Biologics accounted for 38% of U.S. drug spending in 201521. The annual cost for a single biopharmaceutical prescription is typically in the tens of thousands, with the average cost of $16,425 annually for biologics compared to an average of $730 annually for traditional pharmaceuticals as of a 2008 analysis22. Despite biologics being so expensive there has been little evidence to suggest that use of biologics lead to downstream financial offsets; things like reduction in doctors visits or length of hospital stay23.
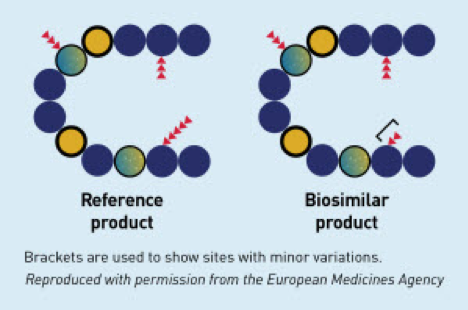
Just as generics provide a lower cost off-brand option to many off-patent pharmaceuticals, so-called “biosimilars” can provide a lower cost alternative to off-patent biologics. A notable difference between generics and biosimilars, however, is that whereas generics contain chemically identical active ingredients compared to the brand name products, biosimilars may not be the exact same molecule as the name brand biologic they were designed to emulate. Because biologics have complex structures, are less stable, and are sensitive to the varying productions processes of different labs, biosimilars will likely consist of a combination of subspecies that may be slightly different than the original producer’s formulation (Figure 2). Developers of biosimilars must demonstrate that although the structure varies slightly compared to the reference product, the biological response remains safe and effective. For this reason and others, developing biosimilars can be somewhat of gamble for biopharmaceutical companies. Biologics demand a high price which represents a large potential reward, however, the initial financial investment required can be quite substantial, representing a large risk. For example, with expensive laboratory supplies and costs associated with obtaining Federal Drug Administration (FDA) approval it can cost $100-250 million to develop a biosimilar24, compared to a measly $1-4 million to develop a new generic. The time for development represents a large investment as well, taking approximately 7 to 8 years to develop a new biosimilar24. Interestingly, federal regulations in the European Union (EU) seem to have made it easier for companies to develop biosimilar products. Because of this, the EU has more data on how much biosimilars might save consumers. Their data suggests that biosimilar products are discounted an average of 15-30% when compared to reference products25. Mulcahy et al. have used such data to predict that Americans could potentially save $54 billion in 10 years between 2017-2026 on their biologics spending by using biosimilars26.
Figure 1: The relative size of a traditional small molecule pharmaceutical, like aspirin, compared to a large macromolecule like a monoclonal antibody, a potential example of a biologic drug27.
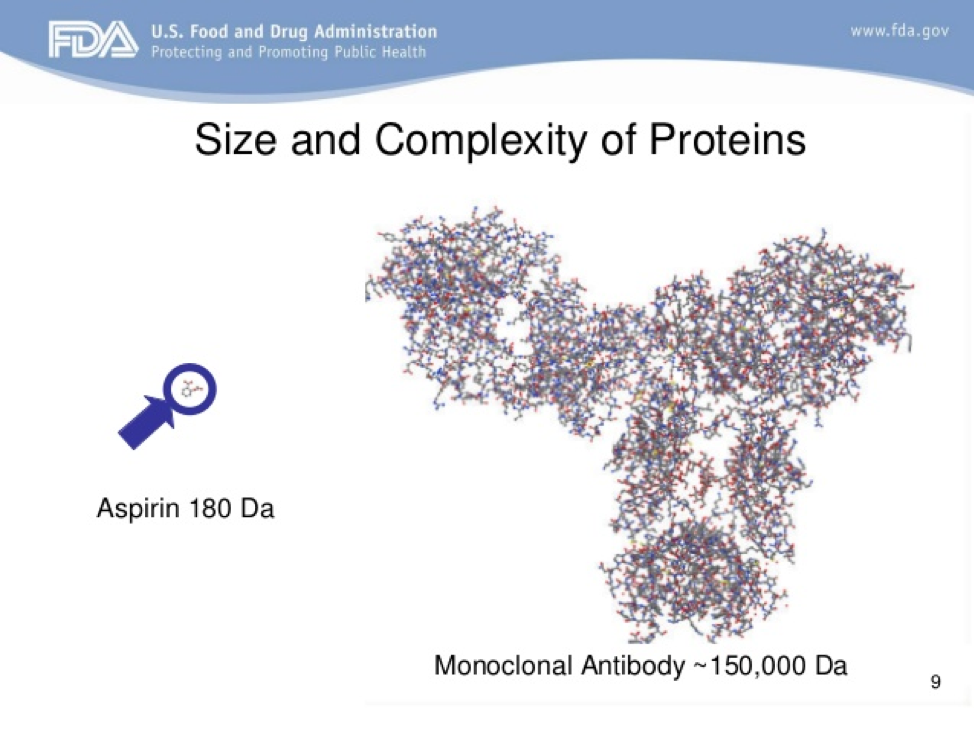
Figure 2: Visual representation of potential minor difference between biosimilar and reference products28.
Repurposing Old Drugs
Repurposing already-approved, off-patent drugs can present an incredible opportunity to lower prescription and healthcare costs. This strategy may be particularly valuable in the developing world since pharmaceuticals companies have little monetary incentive to develop drugs targeted for these areas. Repurposing old drugs can potentially do a great service if they can fill a need in developing countries where new medications are not affordable. In these countries, funds available to spend on healthcare are extremely limited as mentioned earlier. Access to many, especially newer, medications may be very little, in which case people will need to make do with what they have, requiring what has been termed “lean” thinking29. Finding new applications for old, cheap drugs that are on hand can be tremendously innovative and valuable to society.
Repurposing old drugs can potentially be useful to reduce costs in western medicine as well. In particular, it can significantly decrease funds required for companies to get through the FDA approval process. Compared to the roughly $800 million it takes to get a new drug on the market, it only costs $40 million to $80 million for FDA approval of a new use on an old drug30. Additionally, there appears to be great untapped potential for many old drugs. The Repurposing Drugs in Oncology (ReDO) project, for example, has identified 230 drugs (over 75% of which are off-patent) that currently have applications other than cancer but have shown evidence for anti-cancer activity31. Considering the cost of many cancer treatments, repurposing old drugs may have significant financial benefits.
Increasing Shelf Life
In the 1980s the U.S. military had billions of dollars of stockpiled pharmaceuticals that were about to expire and the FDA decided to test batches of the drugs to determine if expiration date extensions were warranted. Upon testing 122 different medications, the FDA determine 90% of them had high enough active ingredients levels to warrant extensions32. In another study of medications that expired 28 to 40 years earlier, 12 of 14 active ingredients were still present at levels 90% of that listed33. Considering the fact that hospitals like Tufts Medical Center in Mass throw out about $200,000 of expired medications per year, extending expiration dates can save hospitals a great deal of money; savings that could then be passed on to patients and insurance companies32. Extrapolate out that example considering there are over 6,000 hospitals in the U.S. and it's not difficult to believe that $2 billion are lost each year in US from expired pharmaceuticals as estimated by Lenzer in 201434.
Inappropriately over-extending expirations is certainly unwise of course, and such extensions are far from all we can do to increase shelf life and save on wasted medications. Additions to formulations to improve stability and optimized storage protocols/technologies are other strategies that can improve shelf life. Biologics and vaccines are much more vulnerable to deterioration and thus have shorter shelf lives as well as stricter storage instructions. For example, according to a Canadian publication, most Canadian vaccines need to be stored between a narrow range of 2°C and 8°C, yet up to 20% of healthcare facilities in Ontario fail to meet this requirement or others35. Additionally, about 4% of vaccines in Ontario expire before they can be used contributing to $3 million in wasted vaccines each year35. Evidently, technologies to improve stability and innovative storage devices may have tremendous cost saving potential.
Production Efficiency
There are potentially many other ways to increase the efficiency of the drug production process in order to lower costs. Just as Baumol explains, other industries have cut costs through automation, and technologies can be developed to help the pharmaceutical and biopharmaceutical industries become more automated. This effort has long been underway with labs integrating robotics for automation starting in the 1980s. One of the most productive uses of automation is in high-throughput screening, the ability to screen many drug candidates at once. Still there is room for increased automation. It’s estimated that with more and optimized automation, drug companies could potentially screen 100,000 compounds per day compared the average 25,000 per week they currently are able to screen36. With that in mind, improvements in automation could potentially save us millions in an industry where it costs close to $1 billion to discover and develop a new drug.
In the biopharmaceutical industry where living organisms are required for the manufacturing process, improvements in growth medias or cellular habitats, for instance, may be an additional line of research to pursue in the effort to increase production efficiency and, thereby, lower costs. Unfortunately, it does not appear there is an incredible amount of research being done in the area. From all the information discussed above, students should have many jumping off points and ideas to be able to start their research and design projects.