Solids, Liquids, and Gases
Matter is made up of very tiny particles known as atoms and molecules.
4
These particles have energy and are in a constant state of motion. The motion and arrangement of the particles that make up a substance determines its state of matter.
3
In a solid the particles are packed tightly together. The energy that bonds these particles together is very strong and prevents the particles from moving around freely. Particles in a solid, as a result, vibrate in their positions.
12
It is these strong bonds that give solids their definite shape and volume.
12
Liquids have particles that are able to move throughout the container that the liquid is in. The bonds that hold the particles of a liquid together are weaker then in solids, which is the reason that particles in a liquid slide and move past one another.
3
Since the particles can move, liquids are able to change their shape and conform to the container that holds them. Their volume however is definite.
Gases are the state of mater that have the weakest bonds between their particles.
12
Particles in a gas are able to move around freely and it is this movement that allows gases to expand or contract. Gases therefore do not have a definite shape or definite volume.
12
|
State of Matter
|
Solid
|
Liquid
|
Gas
|
|
Shape
|
Definite shape
|
No definite shape
|
No definite shape
|
|
Volume
|
Definite volume
|
Definite volume
|
No definite volume
|
|
Particle arrangement
|
Densely packed
|
Close
|
Far apart
|
|
Energy binding particles
|
Very strong
|
Strong
|
Weak
|
Figure 1. State of matter and their properties.
12
Solids are substances that have both definite shapes and definite volumes.
4
A liquid, on the other hand, has a definite volume, but does not have a definite shape. Unlike solids, liquids take on the shape of their container.
3
Gases do not have definite shapes or definite volumes and like liquids gases take the shape of their containers. Since gases do not have a definite volume they can spread out in all directions. Liquids and gases are known as fluids because both substances are able to flow.
Phase Changes
Substances change their state of matter when heat is added or removed.
12
When heat is added particles move faster. This can overcome the bonds holding them together causing a solid to become a liquid. After adding even more heat, the particles spread farther apart and expand, thus becoming a gas, which is much less dense.
12
When heat is removed the opposite happens and particles slow down and move closer together, therefore becoming denser. One exception to this rule of heat affecting density is water. When heat is removed from water it freezes becoming ice. Ice is actually less dense then liquid water. Ice floats on top in a glass of ice water.
There are some positive and negative implications of the fact that ice is less dense than water. When lakes freeze the ice is able to insulate the water below so it does not freeze and fish do not die. If ice sank than the entire lake would freeze each winter. On the other hand, water in cracks on roads freezes in the winter and expands, as this happens over and over, small cracks can become large potholes. Another negative aspect is that when pipes freeze in the winter they can burst. Frost can also wipe out entire crops if an unexpected freeze happens in the late spring.
When heat is added to a solid the particles gain energy and they move around faster and spread further apart. When enough heat is added to a solid it can change into a liquid. This phase change is called melting.
12
Particles in a liquid continue to move even faster and spread further apart when more heat is added. When liquids reach a certain temperature their particles gain enough energy to escape the surface of the liquid and change into a gas. This phase change is called evaporation.
12
When heat is removed from a substance the particles lose energy, slow their motion, and pack together more tightly. Cooling a substance enough can cause a change in its state.
12
Substances undergo condensation when they change from a gas to a liquid.
12
Condensation can be observed on a cold glass of water on a hot summer day. The temperature at which a substance condenses is the same as the temperature at which it boils. When a substances changes from a liquid to a solid, freezing occurs.
12
The temperature at which a substance freezes is the same as the temperature at which it melts.
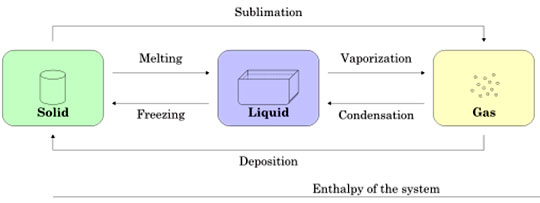
Figure 2. This diagram shows the phase transitions or changes that matter undergoes.
1
(ElfQrin, 2011. Copyright fee licensing under Creative Commons.)
A phase change diagram can be used to show the relationship between temperature and changes of states of matter. During a phase change, the temperature remains constant.
12
The temperature only changes after a substance has changed from one state to another. The temperature will increase or decrease depending on whether heat is being added or removed from the substance. Substances also absorb or release heat at different rates. For example, land absorbs and releases heat faster than water does.
12
Effects of Temperature and Pressure
4, 10
Both temperature and pressure affect the states of matter. In order to discuss what state a specific substances is in temperature and pressure must be specified. Most consider “normal” conditions to be room temperature (20
o
C) and an atmospheric pressure of 1 atm.
10
At normal conditions oxygen is a gas, motor oil is a liquid, and iron is a solid.
10
But materials can exist in different states depending on their temperature and pressure. For example, water can be observed in all three states of matter over the temperature range 0
o
C to 100
o
C at 1 atm pressure.
4
Increasing pressure can liquefy many gases, even if the temperature is well above the normal boiling point. Increasing pressure causes gas molecules to move closer and closer together. When molecules get close enough, “their tendency to behave independently is overcome by the strength of the intermolecular attractions, and the gas condenses into the liquid state.”
10
Butane is a gas at room temperature and 1 atm pressure, but if the pressure is increased butane can also exist as a liquid at room temperature.
10
Some lighters have clear cases that show butane in its liquid state inside the case.
10
When the value to the lighter is opened, the pressure is decreased, and butane comes out as a gas instead of a liquid. Propane is another example of a pressures effect on matter. Propane moves about in its container by when the valve is opened gas is released.
10
Boyle’s Law
The pressure-volume relationship of a gas is known as Boyle’s law.
11
This law states that volume is inversely related to pressure at constant temperature and constant amount of gas. Therefore as volume increase, pressure decreases. Similarly, if the volume decreases, pressure increases.