Karen A. Beitler
Activity 1 –
The size of atoms- drawing paper. Have students write out the number of zeros from meter to nanometer to help them get a sense of the size of an atom. Then have them try to find analogies to help relate better to the comparison of the size of an atom and the size of a polymer. Examples can be found:
http://www.ualberta.ca/~urban/Samples/Peas.htm-
Green Pea Analogy
http://www.newton.dep.anl.gov/askasci/phy99/phy99394.htm -
basketball/atom-dime/earth
http://www.conservapedia.com/Atom -
fly in the cathedral
http://www.agpa.uakron.edu/p16/lesson-print.php?id=nanofiber_chocolate_factory
-Nanometer is one billionth (1 x 10
–9
) of a meter, which can be about 3 to 5 atoms in width.
http://search.yahoo.com/search;_ylt=A0oG7mOvyPJPOj0AFuVXNyoA?p=analogy+for+polymer+size&ei=utf-8&fr=aaplw&xargs=0&pstart=1&b=11&xa=Azc.TeenpHk8uUaR3lYmeQ--,1341397551
-Polymer dimensions
Activity 2 –
Polly's Morning Discovery
Polly is an imaginary teen. She wakes to the sound to her alarm clock (possibly made of polystyrene –a hard low-cost plastic which can be molded into a shape). The sheets and blankets on her bed are most likely made from a combination of a natural polymer (cotton) and polyester like Dacron. Polly gets out of bed her feet landing on a soft carpet. This polymer carpet can be made from a myriad of polymer fibers. This industry is continually looking for new ways to make carpet softer, easier to clean, and aesthetically pleasing to the buyer. Off to the bathroom to brush her teeth Polly finds her toothbrush, made of predominantly nylon, and toothpaste that contains polyethylene glycol, a low-toxicity and low-hazard risk polymer and maybe carboxymethyl cellulose, a polymer thickener, in the medicine cabinet. The cabinet is made of pressed wood fiber (cellulose polymer). As she reaches for the shower door she realizes that it is no longer made from glass but is a clear plastic (acrylic), and the shower bed is also a high-density polymer resin (H.D.P.). In the shower the shampoo and liquid soap (Polyquaternium-10, Polyquaternium-7, Polyquaternium-11, and/or Guar hydroxypropyltrimonium chloride. and silicones that may also be included dimethicone, amodimethicone, cyclopentasiloxane, cyclomethicone, dimethicone copolyols, or dimethiconol) bottles are marked with the HDPE recycling symbol. There is even a plastic squeegee with a note from Mom to wipe down the new shower and spray with a transparent polymer coating (TPC) surface protector. Drying off with a towel (rayon) Polly returns to her room (walking on tile floors made of polymers (PVC) and wood floors (Cellulose) coated with polymers (polyurethane) to put on her clothes, mostly cottons blended with polymer (polyester) for better stretch, resistance to soiling and durability.
Polly heads to the kitchen to make a lunch for school and used a plastic wrap (polyvinylidene chloride) to wrap her sandwich, plastic baggies (LDPE) for snacks and a plastic container (polystyrene) for her muffin. Books & papers are also made of cellulose Polly realizes as she packs them into her book bag. Pouring hot water over a teabag in her Styrofoam cup, she slips into her Crocs (Croclite), puts in her headphones and heads to the bus stop. From music she listens to the seat on the bus and in the classroom, Polly can trace the manufacturing of each product back to a natural or man-made polymer molecule. So, "what's all the fuss about?" she wonders as she walks into her first period class.
1. List 10 polymers that Polly encounters in her morning.
2. Research each polymer and answer 3 questions about each
-
a. What is the polymer made from? (Source material)
-
b. What process that makes this polymer?
-
c. What is the end product of this polymer? (What can it be made into?)
3. Answer Polly's question, "What is the fuss all about?"
Activity 3 –
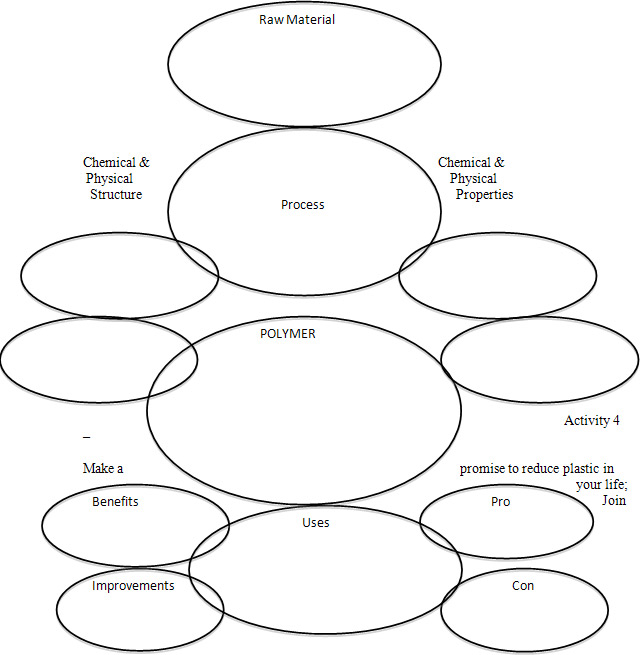
Researching a Polymer
Name ____________________
The polymer to be explored is _________________________________________.
This polymer is used to make _________________________________________.
Chemical formula for this polymer _________________________________________.
Physical structure of this polymer _________________________________________.
Raw material of this polymer_________________________________________.
Chemical properties_________________________________________.
Physical properties_________________________________________.
Process used to make the polymer_____________________________________________
Uses ________________________________________________.
Benefits of the polymer ________________________________________________.
Possible improvements________________________________________________.
Pros of using this polymer________________________________________________.
Cons of using the polymer________________________________________________.
Graphic organizer for Polymer Research
Name __________________
http://5gyres.org/the_5_gyres_plastic_promise
Activity 5 –
Tapped
Worksheet Name_______________________
1. The movie says that by ___________________2/3 of the world will lack access to clean water.
2. Who controls bottle water? _____________________________
3. How much of Earth's surface is covered in water? _______%
4. How much of the Earth's water is drinkable? ____________%
5. Name the 3 largest water bottling corporations __________________________________________________
6. In the next 20-25 years, no matter where you live, clean water access will be an issue due to changes in _________________.
7. _____________ pumped millions of gallons of water from the lake in Atlanta during a level 4 drought in 2007 even though severe restrictions on local residents & businesses were in effect.
8. How do bottled water companies imply tap water isn't healthy? What do they say in their advertisements?
__________________________________________________________________________________________________________________________________________________________
9. How much of bottled water we purchase is just filtered tap water? _______ %
10. Who at the FDA is responsible for overseeing bottled water regulations? ________________
11. Who does the tests on bottled water? ________________
12. How often are Municipal (tap) water sources tested? ____________________
13. Of 80 million single serving plastic bottles used daily, how many end up in landfills? ____________
14. The average world recycling of beverage containers is ________%. The US is _____________%.
15. What is the return rate in Michigan with its 10¢ deposit ____________%?
16. _______________% of Americans don't have curbside recycling
17. In 2008, the Western Garbage patch jumped to _______ times more plastic than plankton?
Name 3 things listed in the credits that YOU can do.
18. ____________________________________________________________________
19. ____________________________________________________________________ 20. ___________________________________________________________________
Activity 6–
5
Most Dangerous Myths of Recycling
found at
http://www.ilsr.org/the-five-most-dangerous-myths-about-recycling/
or at
http://www.grn.com/library/5myths.htm
The Five Most Dangerous Myths About Recycling
-
MYTH #1: We can recycle only 25 to 30% of our solid wastes.
-
MYTH #2: Recycling is more expensive than trash collection and disposal.
-
MYTH #3: Landfills and incinerators are more cost-effective and environmentally sound than recycling options.
-
MYTH #4: Landfills are significant job generators for rural communities.
-
MYTH #5: The marketplace works best in solving solid waste management problems; no public-sector intervention is needed.
Activity 7–
E xamine one aspect of nanotechnology– history, idea, or product
X plain how this technology will affect the future of the planet
P review articles, books, websites, video – LOOK for CURRENT TECHNOLOGY
L ist ideas and sources into a letter– formulate 3 questions to ask
O rganize ideas & sources and connect to CURRENT practice– Write a letter
R ead research and respond what you learn
E ngage others in getting involved through presentation of materials