The Five Main Greenhouse Gases
Gases that contribute to the greenhouse effect include:
Carbon dioxide (CO2)
A minor but very important component of the atmosphere, carbon dioxide, is released through natural processes such as respiration and volcano eruptions and through human activities such as deforestation, land use changes, and burning of fossil fuels. Humans have increased atmospheric CO2 concentration by 47% since the Industrial Revolution began. This is the most important long-lived driving force of climate change.
Processes or regions that predominately produce atmospheric carbon dioxide are “sources”. Carbon dioxide is added to the atmosphere naturally when organisms respire or decompose (decay), during forest fires, when volcanoes erupt and by carbonate rocks formation. Carbon dioxide is also added to the atmosphere through human activities, such as the burning of fossil fuels and forests and the production of cement.
The process of respiration produces energy for organisms by combining glucose with oxygen from the air. Cellular respiration, consumes glucose and oxygen and produces energy and carbon dioxide. The carbon dioxide is released into the atmosphere during the process of cellular respiration, as follows:
C6H12O6 + 6O2 → 6CO2 + H2O + energy
glucose + oxygen → carbon dioxide + water + energy
When organisms die, they are decomposed by bacteria. Carbon dioxide is released into the atmosphere or water during the decomposition process.
An important component of the global carbon cycle is represented by transfer of carbon dioxide from inland waters (rivers, lakes and reservoirs) to the atmosphere. The process is known as carbon dioxide evasion. The global carbon dioxide evasion rate is estimated around 2.1 Pg (petagrams) of carbon per year [4].
Over geologic time, limestone may become exposed (due to tectonic processes or changes in sea level) to the atmosphere and to the weathering of rain. The carbonic acid that forms when carbon dioxide dissolves in water, in turn, dissolves carbonate rocks. This reaction consumes carbon dioxide, as seen in the following two equations:
Carbonate weathering/production
CaCO3 + CO2 + H2O → Ca2+ + 2HCO3-
Silicate weathering (example)
CaAl2Si2O8 + 2CO2 + 3H2O → Al2Si2O5(OH)4 + Ca2+ + 2HCO3-
The clay formation produces carbon dioxide, as seen in the following example:
Al4SI4O10(OH)8 + 3Fe2+ +6HCO3- → Fe3Al2Si4O10(OH)8 + 2Al(OH3) + 6CO2
Carbon dioxide is added to the atmosphere by human activities. When hydrocarbon fuels (i.e. wood, coal, natural gas, gasoline, and oil) are burned, carbon dioxide is released. During combustion or burning, carbon from fossil fuels combine with oxygen in the air to form carbon dioxide and water vapor.
These natural hydrocarbon fuels come from once-living organisms and are made from carbon and hydrogen, which release carbon dioxide and water when they burn.
The basic chemical reaction looks like this:
CH2O + O2 → CO2 + H2O + energy
Not only does the burning of forests release carbon dioxide, but deforestation can also affects the level of carbon dioxide. Trees reduce the amount of carbon dioxide from the atmosphere during the process of photosynthesis, so fewer trees mean more carbon dioxide left in the atmosphere.
Plants and phytoplankton are the main components of the fast carbon cycle. Phytoplankton (microscopic organisms in the ocean) and plants take carbon dioxide from the atmosphere by absorbing it into their cells. Using energy from the Sun, both plants and plankton combine carbon dioxide (CO2) and water to form sugar (CH2O) and oxygen, as shown in the following chemical reaction:
CO2 + H2O + energy → CH2O + O2
Four things can happen to move carbon from a plant and return it to the atmosphere, but all involve the same chemical reaction. Plants break down the sugar to get the energy they need to grow. Animals (including people) eat the plants or plankton, and break down the plant sugar to get energy. Plants and plankton die and decay (are eaten by bacteria) at the end of the growing season. Or fire consumes the plants or stored plant organic matter. In each case, oxygen combines with sugar to release water, carbon dioxide, and energy.
In all four processes, the carbon dioxide released in the reaction usually ends up in the atmosphere. The fast carbon cycle (the movement of carbon through life forms on Earth) is so tightly tied to plant life that the growing season can be seen by the way carbon dioxide fluctuates in the atmosphere. In the Northern Hemisphere winter, when few land plants are growing and many are decaying, atmospheric carbon dioxide concentrations climb. During the spring, when plants begin growing again, concentrations of atmospheric carbon dioxide drop.
Processes or regions that predominately absorb atmospheric carbon dioxide are referred to as sinks. Carbon dioxide may be removed from the atmosphere when it is used by plants and algae for photosynthesis and stored in tissue, dissolved in water, or deposited in the sediments on land or in the ocean (Figure 2).
The average residence time of carbon dioxide in the atmosphere is 4 years.
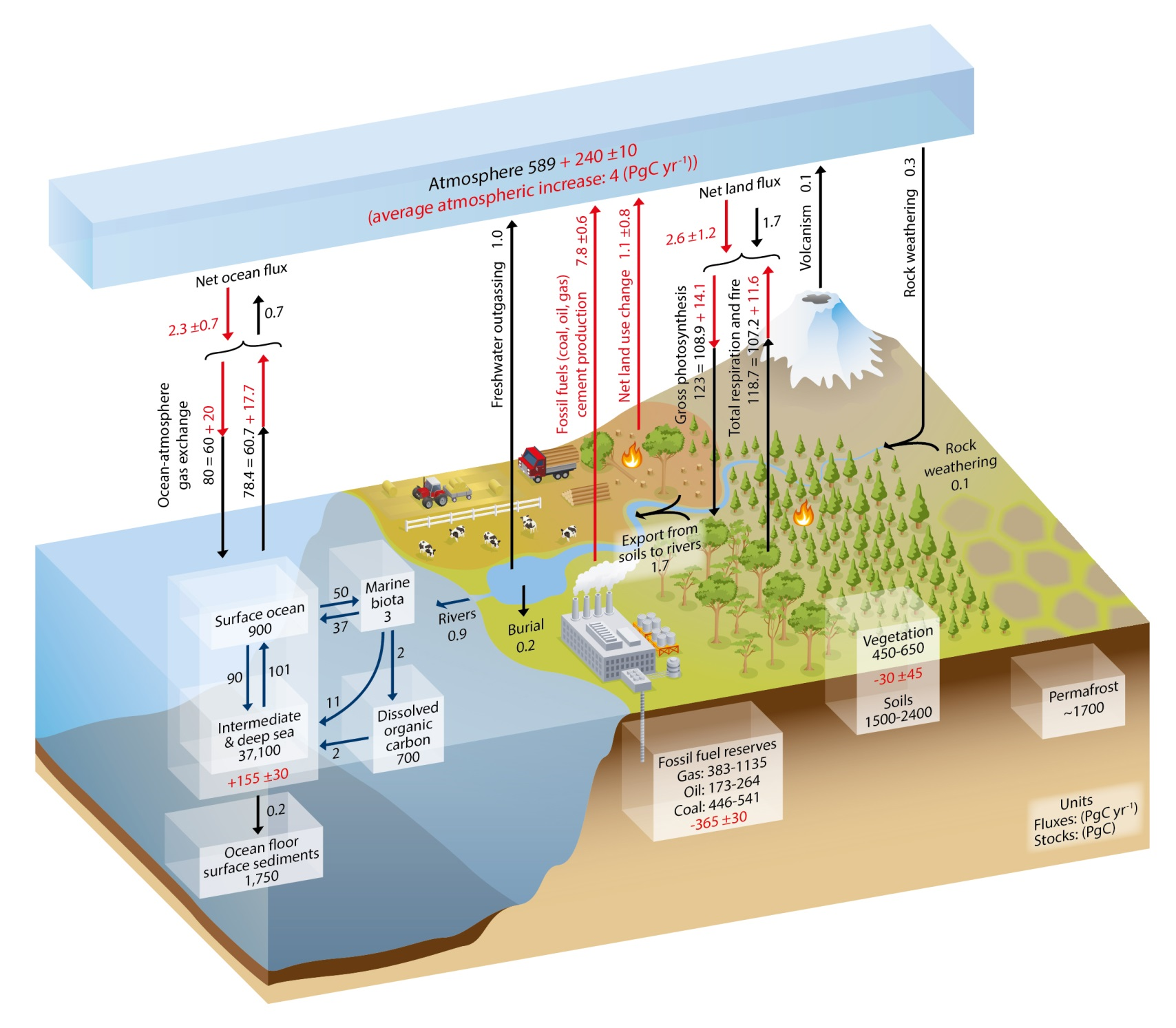
Figure 2. Global Carbon Cycle. Source: https://www.researchgate.net/figure/Simplified-schematic-of-the-global-carbon-cycle-IPCC-2013
Green plants use water from the soil and carbon dioxide from the atmosphere to make carbohydrates (glucose) and oxygen during the process of photosynthesis. In the ocean, algae carry on the same process. During photosynthesis, plants and algae convert the radiant energy of the sun into chemical energy to make carbohydrates (glucose) and produce oxygen as a byproduct. Plants and algae make more glucose in photosynthesis than they consume in respiration. The excess glucose produced by these photosynthetic organisms becomes the food consumed by animals or can be stored as live or dead organic matter and become a carbon sink.
6CO2 + 6 H2O + energy → C6H12O6 + 6O2
carbon dioxide + water + energy → glucose + oxygen
Oxygen is then used by the animals and plants to oxidize this organic matter and produce energy. When animals consume plants or they consume other animals that eat plants, they use the carbohydrates (glucose) as a source of energy.
Carbon dioxide can also be absorbed in the surface water of the ocean. As the concentration of carbon dioxide in the atmosphere increases, some of this carbon dioxide will be dissolved in the oceans. When the water is cooler than the atmosphere, more carbon dioxide is dissolved. Gases are more soluble in cooler water than in warmer water due to the Second Law of Thermodynamics. Gases are exchanged through the ocean surface until equilibrium is reached.
When carbon dioxide combines with water, it forms carbonic acid.
CO2 + H2O → H2CO3
dissolved CO2 + water → carbonic acid
The oceans provide a huge reservoir of carbon. Scientists estimate that the oceans hold more than 50 times the total atmospheric carbon dioxide content. Rainwater dissolves atmospheric carbon dioxide producing carbonic acid. Carbonic acid also reacts with rock through chemical weathering to form bicarbonate ions (HCO3-) that are carried by groundwater and streams to the ocean. Marine organisms use bicarbonate and the calcium (Ca2+) in seawater to produce the calcium carbonate (CaCO3) that they need to make their shells, skeletons, and spines. A coral reef is one example - a coral reef is a huge colony of organisms that use calcium carbonate to build a hard outer skeleton.
When marine organisms die, their remains slowly sink and reach the ocean floor. Over time, these organic materials are compressed by their own weight and other sediments, gradually changing into organic deposits.
The anthropogenic budget of carbon dioxide (Figure 3) can be summarized by the following equation:
Ef + Eluc = Gatm + Socean + Sland + Bim, where
Ef represents the Fossil Emissions
Eluc is the amount of Land Use Change Emissions
Gatm means Atmospheric Growth
Socean represents Ocean Storage
Sland is Land Storage
Bim represents the Imbalance
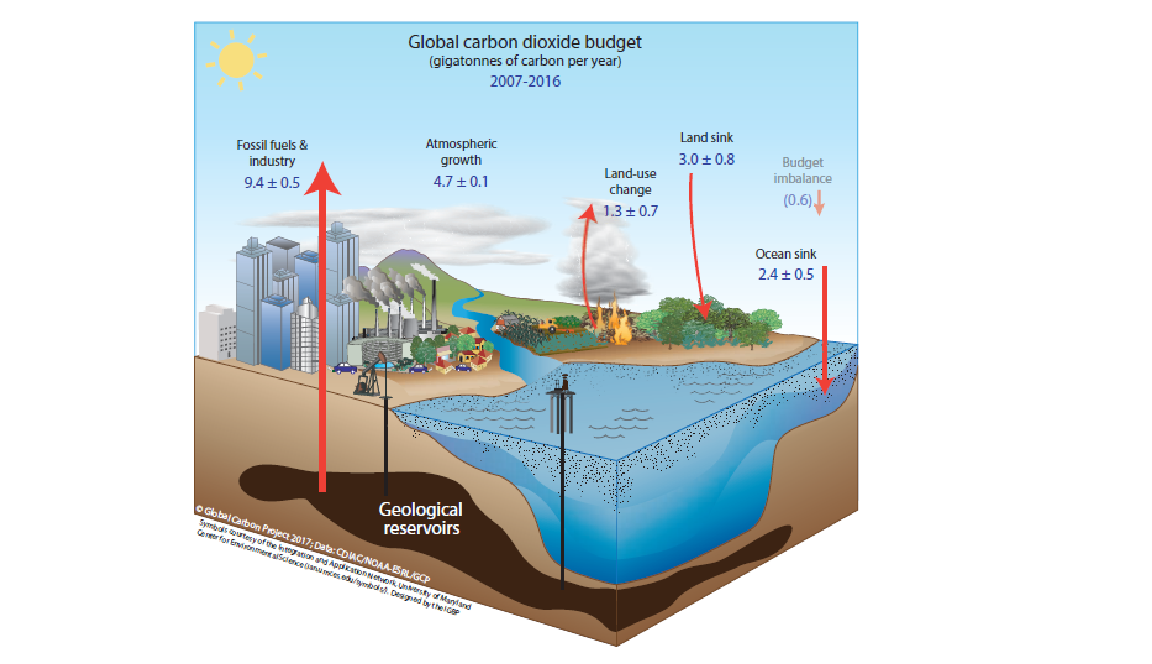
Figure 3. Global Carbon Dioxide budget for 2007-2016. Source: https://www.globalcarbonproject.org/carbonbudget/archive/2017/GCP_CarbonBudget_2017.pdf
Outside of the greenhouse effect, higher atmospheric carbon dioxide levels can have both positive and negative effects on crop yields. Some laboratory experiments suggest that elevated CO2 levels can increase plant growth. However, other factors, such as changing temperatures, ozone, and water and nutrient constraints, may counteract any potential increase in yield. If optimal temperature ranges for some crops are exceeded, earlier possible gains in yield may be reduced or reversed altogether.
Although rising CO2 can stimulate plant growth, research has shown that it can also reduce the nutritional value of most food crops by reducing the concentrations of protein and essential minerals in most plant species. Climate change can cause new patterns of pests and diseases to emerge, affecting plants, animals and humans, and posing new risks for food security, food safety and human health [2].
Methane (CH4)
After carbon dioxide (CO2), methane (CH4) is the second most important greenhouse gas contributing to human-induced climate change. Methane is responsible for 20% of the global warming produced by all greenhouse gases so far. As of 2020, the concentration of methane in the atmosphere is 150% above pre-industrial levels. On a molecule-for-molecule basis, methane is a far more active greenhouse gas than carbon dioxide, but also one that is much less abundant in the atmosphere.
Methane (CH4) is composed of one atom of carbon surrounded by four atoms of hydrogen. Methane is the main component of natural gas. Methane enters the atmosphere and eventually combines with oxygen (oxidizes) to form more CO2. Methane converts to carbon dioxide by this simple chemical reaction:
CH4 + O2 → CO2 + 2H2
methane + oxygen → carbon dioxide + hydrogen
Methane is produced both through natural sources and human activities. It is worth to mention that anthropogenic sources are now greater than natural sources. The natural sources include the decomposition of organic plant and animal matter in such places at wetlands (e.g., marshes, mudflats, flooded rice fields), living plants and termites.
Wetlands are the largest natural global methane source. The resulting global flux range for natural wetland emissions is 153-227 Tg (teragrams) methane per year for the decade of 2003-2012, with an average of 185 Tg methane per year.
The anthropogenic sources of methane include agriculture, and especially rice cultivation, as well as ruminant digestion and manure management associated with domestic livestock, sewage treatment plants, leakage from natural gas pipelines and from oil wells, biomass burning, coal mining and natural gas production.
Biologically, methane is produced via methanogenesis or fermentation by microbes after systems go anoxic (lose all their oxygen), according to the following two equations:
4H2 + CO2 → CH4 + 2H2O
CH3COOH → CO2 + CH4
Methane is broken down biologically by methanotrophs (bacteria that utilize methane as their source of carbon and energy). Methane is also broken down abiotically in atmosphere. In the troposphere, the OH (hydroxyl) radical, produced through a series of reactions that include oxygen, ozone and water, is highly reactive and constitutes the major sink for methane, according to the general reaction:
CH4 + HO- → CH3- + H2O
Permafrost is permanently frozen ground that traps moisture, heat, carbon and produces methane. Permafrost may be as thin as a few meters or as thick as more than 1,000 meters. Vast regions of permafrost in Canada, Alaska, Siberia, and the Tibetan Plateau are starting to thaw. As permafrost melts, carbon dioxide or methane is released, further increasing the concentration of atmospheric greenhouse gases.
In the ocean sediments there are hotspots of thermogenic or biological methane storage. At high pressures and low temperatures of ocean bottom water, methane and water mixtures can for a hydrate or chlathrate. They are fairly stable and can sit around for many centuries. When the hydrate melts, methane is released. The Arctic Ocean’s chlathrates, with higher warming and shallow shelf is particularly worrisome for scientific community.
Methane also contributes to tropospheric production of ozone, a pollutant that harms human health and ecosystems.
The fraction of atmospheric methane reached 1857 ppb in 2018, approximately 2.6 times greater than its estimated pre-industrial equilibrium value in 1750 (Figure 3).
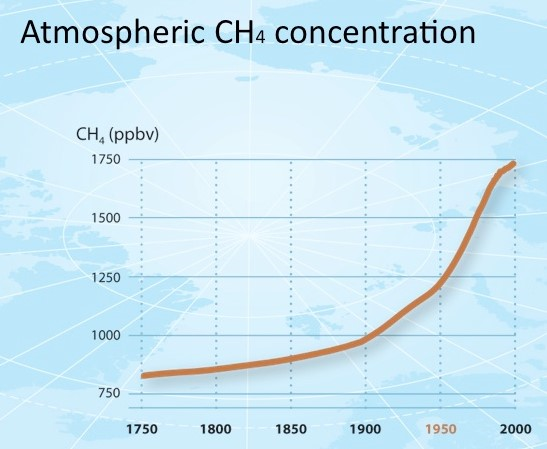
Figure 4. Atmospheric Methane Concentration. Source: https://en.wikipedia.org/wiki/File:Atmosphericmethaneconcentrations.jpg#/media/File:Atmosphericmethaneconcentrations.jpg
Atmospheric methane is a stronger absorber of Earth's emitted thermal infrared radiation than carbon dioxide, as assessed by its global warming potential (GWP) relative to CO2. Although global anthropogenic emissions of CH4 are estimated at around 366 Tg (teragrams) CH4 /year, representing only 3 % of the global CO2 anthropogenic emissions in units of carbon mass flux, the increase in atmospheric CH4 concentrations has contributed approximately 23 % (∼ 0.62 W/m2) to the additional radiative forcing accumulated in the lower atmosphere since 1750 [9].
Nitrous oxide (N2O)
Nitrous oxide (N2O), like carbon dioxide, is a long-lived greenhouse gas that accumulates in the atmosphere. Over the past 150 years, increasing atmospheric N2O concentrations have contributed to stratospheric ozone depletion and climate change, with the current rate of increase estimated at 2 per cent per decade.
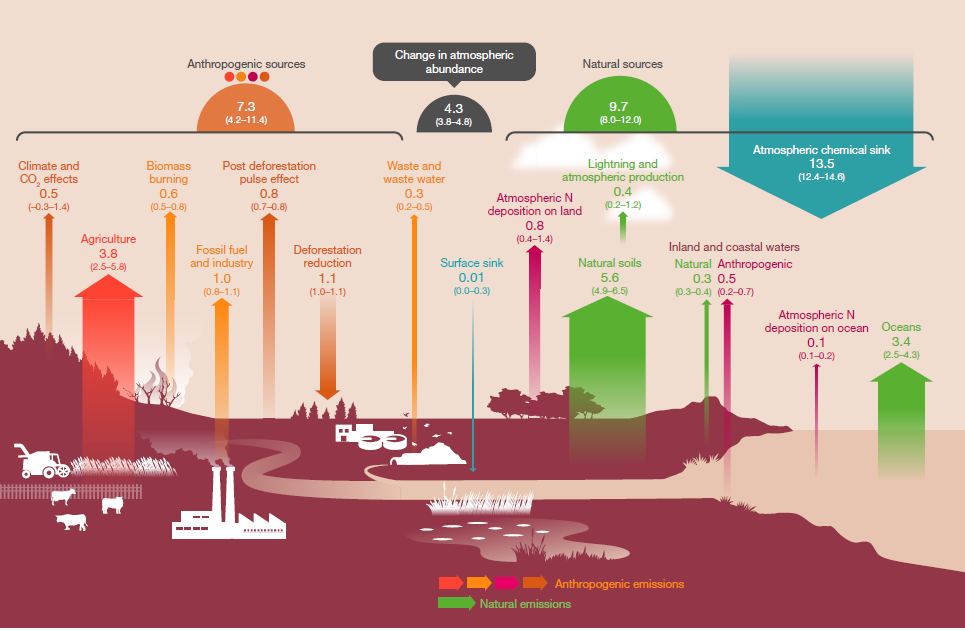
Figure 5. Global Nitrous Oxide budget for 2006-2017. Source: https://www.globalcarbonproject.org/nitrousoxidebudget/
Nitrous oxide is produced by soil cultivation practices, especially the use of commercial and organic fertilizers, fossil fuel combustion, nitric acid production, and biomass burning.
Nitrate (NO3-) and ammonia (NH3) are used as fertilizers. Bacteria convert a small amount of this nitrate and ammonia into the form of nitrous oxide as byproducts of the nitrification and denitrification processes, as follows:
2NH3 + 3O2 → 2NO2 + 2H+ + 2H2O
NO3- + 2H+ + 2e- → NO2- + H2O
NO2- + 2H+ + 2e- → NO + H2O
2NO + 2H+ + 2e- → N2O + H2O
N2O + 2H+ + 2e- → N2 + H2O
Internal combustion engines also produce nitrous oxide.
Nitrous oxide has a long residence time in the atmosphere, an average of about 120 years.
Global nitrous oxide emissions were around17 teragrams of nitrogen per year between 2007 and 2016 (Figure 5). Global human-induced emissions, which are dominated by nitrogen additions to croplands, increased by 30% over the past four decades to 7.3 teragrams of nitrogen per year. This increase was mainly responsible for the growth in the atmospheric burden.
Studies point to growing nitrous oxide emissions in emerging economies - particularly Brazil, China and India. Analysis of process-based model estimates reveals an emerging N2O - climate feedback resulting from interactions between nitrogen additions and climate change. The recent growth in nitrous oxide emissions exceeds some of the highest projected emission scenarios underscoring the urgency to mitigate N2O emissions [10].
Chlorofluorocarbons (CFCs)
Synthetic compounds entirely of industrial origin are used in a number of applications, but now are largely regulated in production and release to the atmosphere by international agreement for their ability to contribute to destruction of the ozone layer. They are also greenhouse gases.
Halocarbons, which are composed of carbon, chlorine, fluorine, and hydrogen, include chlorofluorocarbons (CFCs). Chlorofluorocarbons are synthetic gases that were used in cleaning solvents, refrigerants, and plastic foam.
Chlorofluorocarbons are tightly bound, nonreactive molecules. They are not soluble in water. They do not absorb visible or near-ultraviolet radiation. As a result of these properties, chlorofluorocarbons have long atmospheric residence times (ranging from 50 to several hundred years) and are able to provide their constituent atoms to the stratosphere.
In the late 1980s, the Montreal protocol led to a drastically reduction in CFC production. Consequently, the atmospheric concentrations of CFCs leveled off. Presently, the atmospheric concentrations of CFC11 and CFC 113 are decreasing significantly. The atmospheric concentration of CFC12 has reached a plateau and will drop at a lower rate in the future.
Water vapor
Water vapor is the most abundant greenhouse gas, but importantly, it acts as a feedback to the climate and it has no direct anthropogenic source. Water vapor increases as the Earth's atmosphere warms, but so does the possibility of clouds and precipitation, making these some of the most important feedback mechanisms to the greenhouse effect.
If there is too much water vapor in the air, it will condense out as rain. Conversely, if the air is extremely dry, any available liquid water will tend to evaporate into it. Water vapor is also involved in a positive feedback loop acting on global temperature. Because warmer air holds more water vapor than cooler air, warming allows more water to evaporate before it rains. The water vapor feedback is powerful enough to more or less double the climate impact of rising carbon dioxide concentration. If it were not for the water vapor feedback, Earth’s climate would be much less sensitive to carbon dioxide and maybe we would not be worrying so much about global warming [3].
Water vapor molecules absorb some shortwave radiation. Buildup of water vapor in the atmosphere leads to global dimming, that is, reduction of solar radiation at the Earth’s surface. The rate of reduction is about 1 to 3 W/m2K. As a result of more water vapor in the atmosphere, there will be more longwave radiation from the atmosphere to the Earth’s surface [2].
The rate of increase of longwave radiation received at the surface is about 6 W/m2K. Another consequence of water vapor buildup is that rain intensity will increase. Many people estimate that individual rain intensity will increase at the rate of about 7% per Kelvin.
Global Warming Potential
Greenhouse gases (GHGs) warm the Earth by absorbing energy and slowing the rate at which the energy escapes to space; they act like a blanket insulating the Earth. Different GHGs can have different effects on the Earth's warming. Two key ways in which these gases differ from each other are their ability to absorb energy (their "radiative efficiency" - related to how they interact with longwave radiation and their concentration in the atmosphere), and how long they stay in the atmosphere (also known as their "lifetime").
The Global Warming Potential (GWP) was developed to allow comparisons of the global warming impacts of different gases. Specifically, it is a measure of how much energy the emissions of 1 ton of a gas will absorb over a given period of time, relative to the emissions of 1 ton of carbon dioxide (CO2). The larger the GWP, the more that a given gas warms the Earth compared to CO2 over that time period. The time period usually used for Global Warming Potentials is 100 years. GWPs provide a common unit of measure, which allows analysts to add up emissions estimates of different gases and allows policymakers to compare emissions reduction opportunities across sectors and gases [11].
Carbon dioxide, by definition, has a GWP of 1 regardless of the time period used, because it is the gas being used as the reference. Carbon dioxide remains in the climate system for a very long time: emissions cause increases in atmospheric concentrations of CO2 that will last thousands of years.
Methane (CH4) is estimated to have a GWP of 28-36 over 100 years. A methane molecule is 30 times stronger than a molecule of carbon dioxide (CH4 absorbs much more energy than CO2), but methane is present in smaller concentrations and has a shorter lifetime than carbon dioxide. The atmospheric life time of CH4 is 9 ± 2 years, making it a good target for climate change mitigation. The net effect of the shorter lifetime and higher energy absorption is reflected in the GWP. The methane’s GWP also accounts for some indirect effects, such as the fact that CH4 is a precursor to ozone, and ozone is itself a greenhouse gas.
Nitrous Oxide (N2O) has a GWP 265–298 times that of carbon dioxide for a 100-year timescale. N2O emitted today remains in the atmosphere for more than 100 years, on average.