Victoria A. Raucci
If you ask students what is the color of light, most students respond that it is "white" or "clear". When first asked why an apple is red, the most common answer is that there is red dye in the skin of the apple. Both of these popular misconceptions are difficult to explain to students who are at an age where they are transitioning from the concrete operational stage of development (ages 7 to 11) to the more abstract thoughts in the formal operational stage (beginning at age 12), according to Jean Piaget's stages of development. Hence, it is important to first understand the initial concepts related to light and color before we can create inquiry-based lessons on which students can explore and grasp the concept of why colors appear the way they do.
Our modern understanding of light and color began in the 1660s when Sir Isaac Newton refracted white light into a prism, which separated the light into the colors of the visible light spectrum: Red, orange, yellow, green, blue, indigo, and violet (We often teach children an acronym to help them remember these colors: ROY G. BIV). When Newton first demonstrated that the prism could separate the colors of white light, he had to dispel the theory that the prism "created" the colors. He was able to do this by reflecting the scattered beams into another prism, proving that the separate colors came together to once again create white light.
44
So if all objects are illuminated by white light, we now ask the question: Why do colors appear the way they do? All colors are absorbed into an object except for the one color that reflects off of it. For example, a blue object exposed to white light will absorb most of the wavelengths except those related to the blue light. The blue wavelengths are reflected off the object. (See the illustration in figure 2)
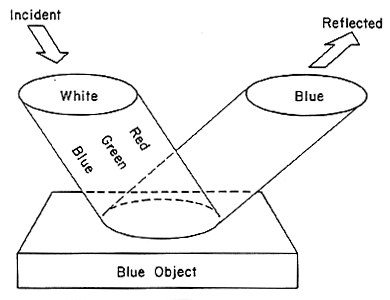
Figure 2: How light is reflected off of an object
55
(figure 2 from http://www.optics4kids.org/home/teachersparents/articles/color-and-light/)
Students often ask what makes some colors absorb and others reflect. In every object, there are atoms and molecules, which contain electrons. The electrons vibrate at specific frequencies. Similar to a tuning fork or a musical instrument, the electrons of atoms have a natural frequency at which they vibrate. When a light wave with that same natural frequency comes upon an atom, then the electrons of that atom will vibrate. If a light wave of a given frequency strikes a material with electrons having the same vibrational frequencies, then those electrons will absorb the energy of the light wave and will be put into motion. As a result, the light wave with that frequency is absorbed by the object and is not released in the form of light. It is when frequencies of light waves do not match the frequencies of the vibration of the objects that the object will reflect the light wave of the color associated with it.
66
Although it is interesting that scientists have learned what makes colors appear the way they do, studies continue to focus on how light and color can be harnessed to perform new tasks. The development of fiber optics as telecommunication; the use of lasers for their precision in surgical techniques, as well as in military targeting; the psychological uses of color to stimulate thoughts and feelings lead us to ask new questions: What other uses are there for light and color that we have not yet discovered? We already know that radiation can be used to treat cancer, ultraviolet light can kill bacteria, and blue light can benefit jaundiced newborns, but can light destroy viruses? What are the other possibilities that color can be used for, outside of aesthetic purposes? If we explore these possibilities with our students, we might be able to encourage them to think of new ways to utilize the properties of light and color.