Chemical Reactions
A chemical reaction is process that changes one set of chemicals into something new with a new set of properties. Chemical reactions can involve putting things together or breaking them apart. Everything that happens in living things is based on chemical reactions that occur in our cells
1
. These chemical reactions involve making and breaking chemical bonds, both of which use energy. All living organisms need energy to carry out the chemical reactions in their cells in order to stay alive. The energy needed to start these reactions is called activation energy
1
. Enzymes are known as "natures catalysts" and help to lower the activation energy needed to start chemical reactions in living things
1
. The image below shows how the energy needed to start a chemical reaction is lowered by the presence of an enzyme catalyst.
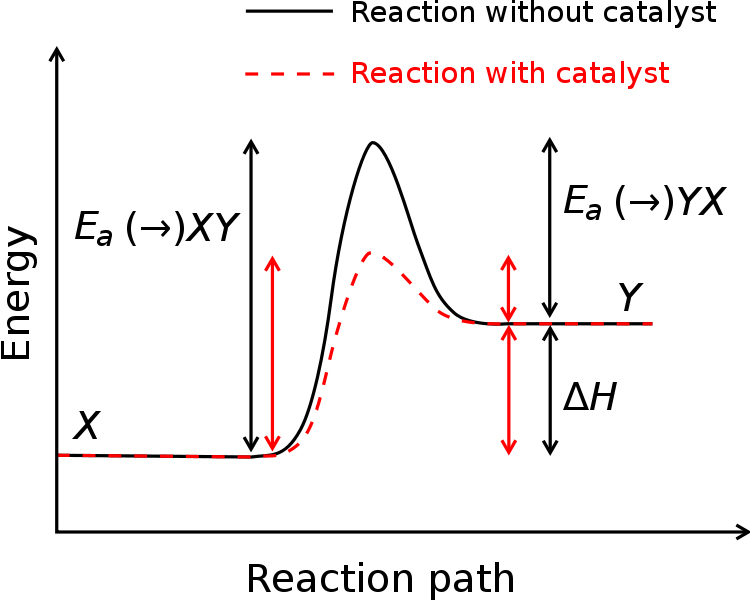
http://en.wikipedia.org/wiki/File:Activation_energy.svg
The function of enzymes is explored in greater depth in the following section.
Enzymes as Catalysts
Enzymology is the study of enzymes and is a fundamental concept in biochemistry
2
. The study of enzymes began in the early 19
th
century with research on fermentation and digestion
2
. Catalysts are substances that speed up chemical reactions
1
. Enzymes are biological molecules that act as catalysts to speed up chemical reactions in the living organisms; without the presence of enzymes, these chemical reactions would proceed too slowly
1
. In fact, at any given time, all of the work being done in our cells is being catalyzed by enzymatic reactions
3
. Enzymes are created by cells and are necessary for all of the complex reactions that make life possible
4
. It is estimated that cells contain over 3000 different enzymes, each of which catalyze different chemical reactions
5
. Most enzymes are made of protein and can also include other small molecules such as RNA
4
Since enzymes are proteins, they are made up of amino acids, which impact the shape and structure of the enzyme
3
. The impact of the amino acids on enzyme shape will be discussed in more detail later in this paper.
Enzymes function by binding a specific substrate and speeding up the chemical process of creating a product. Their purpose is the speed up the process of putting things together or taking them apart in your cells, allowing them to grow and reproduce
3
. Voet and Voet outline four characteristics of enzymatic reactions that make them different from other chemical reactions: higher reaction rates, milder reaction conditions, greater reaction specificity and capacity for regulation
3
.
Reactions that are catalyzed by enzymes are typically 10
4
to 10
12
times faster than uncatalyzed reactions, allowing for greater product output by several orders of magnitude
2
. Additionally, enzymatic reactions can occur under mild reaction conditions, such as lower temperature and pressure and neutral pH, when compared to uncatalyzed reactions
3
.
From the early research of enzymatic reactions, the lock-and-key hypothesis was developed. This hypothesis states that, "the specificity of an enzyme (the lock) for its substrate (the key) arises from their geometrically complementary shape"
2
. Substrates are the reactants in a chemical reaction and enzymes have a great specificity for their substrates
2
. The enzyme and substrate are structurally compatible and fit much like a lock and key or puzzle pieces
5
. Enzymes also have a great specificity for their products, rarely creating side products or errors, making them extremely efficient in catalyzing chemical reactions
2
. Enzymes are also able to self-regulate their catalytic activity based upon the concentration of substrates and products
2
.
The basic action of an enzyme is to have the substrate bind to a specific site on the enzyme, known as the active site, where the substrate is then put together or taken apart
3
. This basic action can be broken down into four simple steps: (1) an enzyme and substrate are in the same vicinity in a cell (2) a substrate binds to the enzyme at the active site (3) the substrate is broken down or combined through a process called catalysis (4) the enzyme releases the substrate
6
. When the substrate has bound to the enzyme at the active site this is called the enzyme-substrate complex. The diagram below illustrates the basic action of an enzyme.
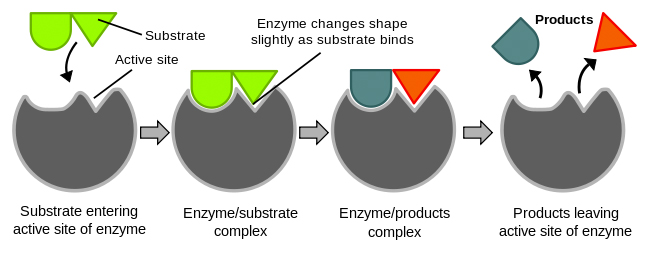
http://en.wikipedia.org/wiki/File:Induced_fit_diagram.svg
General Classes of Enzymes
There are hundred of enzymes at work in the cell at any given time, but they can be classified generally into groups based on their functions.
There are several classes of enzymes that break down biological materials. Proteases and peptidases are enzymes that break down proteins and peptide chains into their individual amino acids
3
. This class of enzymes can be very specified to only break down certain proteins, while others are generalized and will break down any protein
3
. Amylases are enzymes that break down starch and sugar into smaller molecules
3
. Cellulases also break down cellulose into simple sugars
3
. Another class of enzymes used in the breakdown of materials are lipases, which break down fats
3
.
Aside from breaking molecules down, there are other classes of enzymes that perform cellular processes to make energy in the form of ATP
3
. There are 10 enzymes that allow cells to perform glycolysis and another eight enzymes that control the citric acid (Krebs) cycle, both of which work together to create ATP for our cells to use
3
.
Additionally, there are groups of enzymes that work on DNA. Restriction enzymes recognize patterns in DNA and cut DNA chains
3
. Other enzymes, such as DNA polymerase and DNA-binding proteins work to reproduce and block access to DNA, respectively
3
.
There are even enzymes that produce other enzymes! Virtually all cellular processes require enzymes to make the chemical reactions occur properly.