Like carbohydrates, lipids (fats, oils and waxes) are also made of hydrogen, oxygen, and carbon combined into molecules called fatty acids. These fatty acids are linked to glycerol molecules.
Unlike carbohydrates, lipids do not readily combine with water. Some fats are solid (body fat), some are liquid (oils on our skin). These chemical properties are a result of the specific fatty acid they contain.
Fats are triglyceride molecules. They have three fatty acid molecules bonded to a glycerol molecule and are typically solid at room temperature. Fats are very energy dense molecules: they have nine calories per gram.
Oils (a kind of fat) are liquid at room temperature and can be made from living things, typically plant material. Fossil fuel oil refers to oils that had been made by plants millions of years ago and were buried.
Waxes are fatty acids linked to alcohol molecules and have a low melting point. They are synthesized by both plants and animals.
Butter is a combination of butterfat, proteins and water. It is solid at room temperature and below because the fatty acid chains are closely packed and the strands are weakly attracted to each other.
Margarine is made from hydrogenated vegetable or animal fat with skim milk. Hydrogenation involves the addition of hydrogen to the oil in order to straighten out the fatty acid chain and make it solid at room temperature
Figure 2: Phospholipid
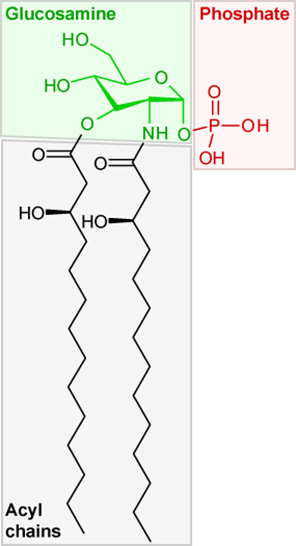
Lipids, such as the phospolipid in figure 2, are essential for the assembly of cell membranes since they are generally not water soluble. Cell membranes allow for the separation of cells as well as spaces within a cell, which allow for chemical reactions. If all the water soluble elements within a cell could freely mix the complex work of living things could not properly occur. It would be chemical chaos within the cell.
In figure 2 the structure of the phospholipid molecule is very important to the function of the molecule. As with the other lipids we have surveyed, this phospholipid has a long actyl "tail". This is segment of the molecule is repelled by water (hydrophobic) and the glucosamine and phospate "head" of the molecule has an affinity for water.
When these molecules self assemble into a membrane – either as a cell membrane or an organelle membrane, they arrange themselves into a two molecule thick sheet. In this arrangement the acyl "tails" point inward, toward each other, and the glucosamine and phospate "heads" point outward.