With a sudden warming of the atmosphere, surface, and ocean, there must be a cause of why this is all happening within the past century and why it’s so much more drastic within the past twenty years. The atmospheric concentrations of carbon dioxide (CO
2
), methane (CH
4
), and nitrous oxide (N
2
O) have all increased to levels not observed in over eight hundred thousand years.
3
Carbon dioxide levels have increased by 40% since pre-industrial times. This is primarily attributed to the burning of fossil fuels. Methane and nitrous oxide have each increased by 150% and 20%, respectively. These historical comparisons are made possible by taking samples from ice cores. In artic regions where ice has been consistently frozen, scientists have drilled far enough down and retrieved samples of ice that froze eight hundred thousand years ago. In those ice crystals are gaseous pockets of past atmospheres. The deeper within an ice core a sample is taken, the older it is. As new snow and ice accrues, over time, more snow and ice will accumulate over it, making a new layer. Over the course of several thousand years, ice crystals become buried, but by drilling and collecting samples, accurate estimates of air quality from the pase can be made.
From 1750 to 2011, the amount of carbon dioxide released into the atmosphere has been estimated. In that time span, we’ve released about 375 Gt of carbon into the atmosphere produced by fossil fuel combustion and cement production. Deforestation and other land use change has also added about 180 Gt of carbon into the atmosphere as well. The planet has found its own ways to reabsorb the carbon that is being released into the atmosphere. Out of the 555 Gt of carbon released, approximately 155 Gt of carbon has been taken back into the ocean and 160 Gt of carbon has accumulated in natural terrestrial ecosystems. All of that carbon going back into the ocean isn’t beneficial. It decreases ocean pH. With more carbon dioxide, the oceans undergo a process called “acidification” where the carbon dioxide and water chemically combine to create carbonic acid, a weak acid, but when 155 Gt of carbon is added to the ocean, it’s enough to decrease the ocean’s pH by 0.1.
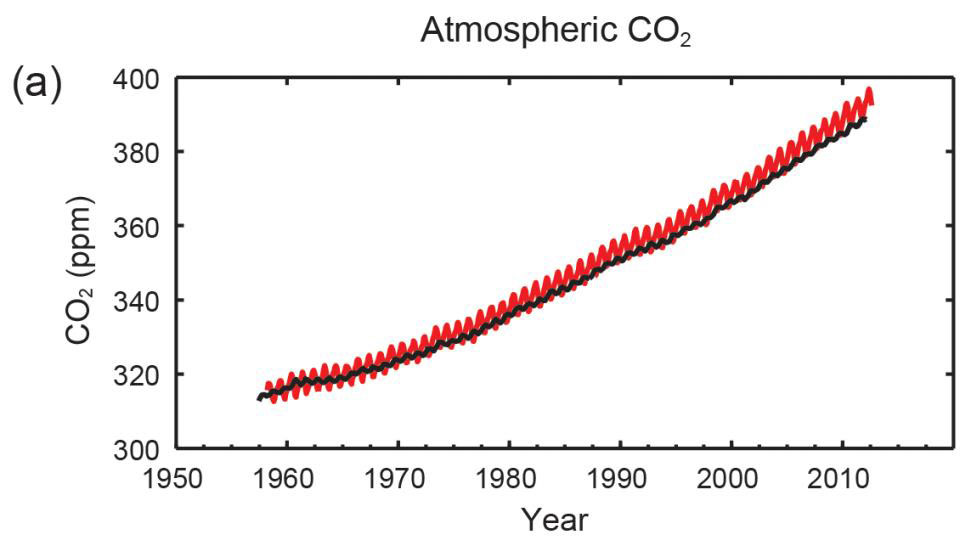
Figure 1: Multiple observed indicators of a changing global carbon cycle: (a) atmospheric concentrations of carbon dioxide (CO2) from Mauna Loa (19°32’N, 155°34’W – red) and South Pole (89°59’S, 24°48’W – black) since 1958. Full details of the datasets shown here are provided in the underlying report and the Technical Summary Supplementary Material. {Figures 2.1 and 3.18; Figure TS.5} (IPCC, 2013)