Huwerl Thornton, Jr.
Water is the most influential element of nearly all foods. It is also used in a way in which food can be heated so that it can change the flavor, texture, and stability. One particular property of water solutions, their acidity or alkalinity, is a source of flavor, and has an important influence on the behavior of other food molecules.
14
Water is the most familiar chemical known. It is the smallest and simplest of all of the basic food molecules. It has just three atoms, two hydrogen and one oxygen. Water is probably the most important element on the planet. All life on the planet, including our own lives exists in a water solution. The human body is made up of about 60% water by weight, raw meat is about 75% water and fruits and vegetables are anywhere up to 95% water.
15
Each water molecule is polar, which means it is electrically asymmetric. A water molecule has a positive end and a negative end. This happens because the oxygen atom exerts a stronger pull than the hydrogen atoms on the electrons they share. As a result, because the hydrogen atoms project from one side of the oxygen, it forms a V shape. The water molecule now has an oxygen end and a hydrogen end. The oxygen end is more negative than the hydrogen end. This polarity means that the negative oxygen on one water molecule feels an electrical attraction to the positive hydrogens on other water molecules.
16
This attraction brings the two molecules closer to each other and holds them there. This is called a hydrogen bond. The molecules in ice and liquid water are participating in from one to four hydrogen bonds at any given moment.
17
The hydrogen bonds in liquid water are transitory, they are constantly being broken and reformed. This happens because the motion of the molecules in liquid is forceful enough to overcome the strength of the hydrogen bonds.
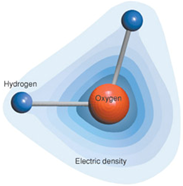
Here is a diagram of a water molecule courtesy of the Lawerence Livermore National Laboratory website: https://www.llnl.gov/str/October05/Mundy.html. It does a nice job of showing the bond between the positively charged hydrogen and the negatively charged oxygen.
McGhee has a small section in his book entitled: Water Is Good At Dissolving Other Substances. This is essential to the theme of this unit. The general person usually thinks of water if something needs to be dissolved. Let's look at why water is good at dissolving other substances. Water forms hydrogen bonds not only with itself, but with other substances that have at least some electrical polarity, some unevenness in the distribution of positive and negative electrical charges.
18
Out of all of the major food molecules, carbohydrates and proteins, which are much larger and much more complex than water, have polar regions. This is significant because water is attracted to these polar regions and they cluster around them. By doing this, the water surrounds the larger molecules and separates them from each other. When each of the larger molecules is mostly surrounded by water molecules, then that substance is considered to dissolved. This is important for small molecules like sugar and salt. This is a crucial concept for students to understand in this unit. This will be a critical element in the upcoming lessons. As they are making KOOL-AID® for solutions and JELL-O® for gels this is a vital step in the process. This will also be essential during one lesson when they are comparing whether the liquid they see is a solution or if it is a suspension.
The hydrogen bonds in water have a strong effect on how water absorbs and transmits heat. Water exists as a solid at low temperatures 32
o
F and below, a liquid at temperatures of 33
o
F to 211
o
F, and a gas at a high temperatures of 212
o
F and higher. The hydrogen bonding between the molecules has an effect on all three phases. Water as a solid is commonly referred to as ice. Ice happens when the attraction of the molecules for each other becomes stronger than their movements. The molecules settle into a compact arrangement determined by their geometry.
19
As a result, ice is a solid that has more space between molecules than the liquid phase thus, it expands. When raw plants or meats are frozen, the tissues are damaged and this causes liquids to leak when they are thawed. While the raw vegetables or meat is freezing, the expanding ice crystals rupture the cell membranes. Thus, when those vegetables or meat begins to thaw, they lose their internal fluids.
As stated earlier, the hydrogen bonds in water play an important role in the three phases of water. The hydrogen bonds in water cause water to have a high specific heat. Specific heat is the amount of energy that is needed to raise the temperature by a given amount. What this means is that water has to absorb a lot of energy before its temperature rises. Thus, liquid water is slow to heat up. It takes 10 times the energy to heat an ounce of water 1
o
as it does to heat an ounce of iron 1
o
.
20
As a result, a pan on the stove with nothing in it will get hotter much quicker than water that may also be in a pan on the same stove with the same heat. The reason why water heats slower than metal is that before the heat energy added to the water can cause molecules to move faster and its temperature to rise, some of the energy must first break the hydrogen bonds so that the molecules can move faster. This is the boiling that can be seen when water is heated. This also allows the water to hold a higher temperature for a longer period of time.
As water vaporizes and turns into steam, it absorbs a lot of heat. The hydrogen bonding gives water an unusually high latent heat of vaporization.
21
This means that the amount of energy that water absorbs without a rise in temperature as it changes from a liquid to a gas. This is important to people because this is the process that helps us from overheating. Sweating uses this principle by using the water that has collected on our overheated bodies. The water on the skin collects and absorbs energy/heat and carries it away into the air. This process has been used even in ancient cultures. Porous clay pots were used to keep water or wine cool. The porous clay pots would evaporate moisture continuously and keep its contents cool. Cooks also use evaporation in cooking foods like custards when they partially immerse the containers in open water. Oven-roasted meats cooked slowly also use this process. Evaporation allows the food to release energy or it removes energy from the surrounding area and this causes the food to cook more gently.
When steam condenses into water, it releases a lot of energy. When steam hits a cool surface and condenses into liquid water, it gives that same high heat of vaporization.
22
As a result of this, steam is a very effective and quick way to cook food. Steam at 100
o
C/212
o
F is very different than air at the same temperature which is also a gas. This explains why it is much easier to tolerate the heat in an oven for a much longer time than being exposed to steam at the same temperature. Steam will scald or burn skin within seconds as opposed to air which will take a much longer time to achieve the same effect. This is why it is very important to take safety precautions when working with steam. It can be much hotter than boiling water and can burn within seconds.