The Gay-Lussac law
states that at a
constant volume (V=constant),
the pressure of a gas is directly proportional to its absolute temperature as shown in the first figure above: The pressure of a gas increases as the temperature of that gas increases and the pressure decreases when the temperature decreases.
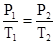
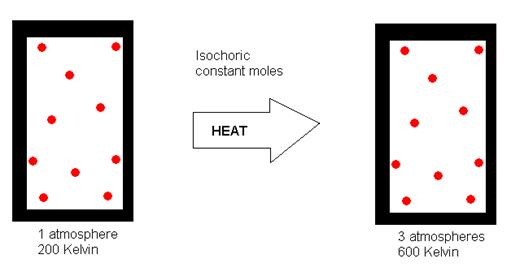
The kinetic molecular theory of gases accounts for this behavior because when the temperature of a gas increases, the speed of its particles increases, the particles hitting the wall with greater force and greater frequency. Since the volume remains the same, this would result in increased gas pressure, as depicted in the following illustration (where “isochoric” means “constant volume”).
Source: http://dlrgenchem.com/LECTURES/Gaslaw.htm