The first thermometers were glass and contained alcohol, which expands and contracts as the temperature changes. Alcohol has one of the largest coefficients of volumetric thermal expansion (0.00149/ºC). It is 8.3 times larger than that of mercury (0.00018/ºC) and 7.0 times greater than water’s coefficient (0.000214 /ºC).
The German scientist Daniel Fahrenheit started his work in thermometry as early as 1706 and at first he used tubes filled with alcohol only, but after few years he used tubes filled with mercury. One reason is that mercury can be used to measure temperatures above the boiling point and below the freezing point of water. Another reason is that mercury, due to its surface tension, does not stick to the tube as the alcohol does, thus making the readings more accurate. For eighteen years, Fahrenheit kept his method to manufacture thermometers a secret, for commercial reasons, but between 1724 and 1726 he published five brief papers in the “Philosophical Transactions”, a scientific journal from The
Royal Society
(England). In the fifth paper, Fahrenheit describes the way he used to build his thermometers [5]:
“The scales of thermometers begin with 0
o
and go to 96
o
. The division of the scale depends upon three fixed points which are obtained in the following manner: the first point below, at the beginning of the scale, was found by a mixture of ice, water and sal-ammoniac (ammonium chloride) or also sea salt; when a thermometer is put in such a mixture the liquid falls until it reaches a point designated as a zero. […] The second point is obtained when water and ice are mixed without the salts named; when a thermometer is put into this mixture the liquid stands at 32
o
and this I call the commencement of freezing, for still water becomes coated with a film of ice in winter when the liquid in the thermometer reaches that point. The third point is at 96
o
; the alcohol expands to this height when the thermometer is placed in the mouth, or the arm-pit, of a healthy man and held there until acquires the temperature of the body.”
The freezing point of water is 32 °F and the boiling point is 212 °F on this scale. The Fahrenheit scale is typically not used for modern scientific purposes.
The Celsius scale of the metric system is named after Swedish astronomer Anders Celsius (1701-1744). The basis of Celsius scale is represented by the freezing and boiling points of water at 0°C and 100°C respectively. The distance between those two points is divided into 100 equal intervals, each of which is one degree. An outdated term sometimes used for the Celsius scale is “centigrade” because there are 100 degrees between the freezing and boiling points of water on this scale. However, the preferred term is “Celsius.”
The two scales are related using the equation: T(ºF ) = T(ºC ) × 9/5 + 32
The Celsius and Fahrenheit scales were based on the properties of a particular substance (water) under particular circumstances (normal atmospheric pressure on Earth). A scale that has its basis in much more fundamental physics is the Kelvin scale, first proposed in the nineteenth century.
The Kelvin temperature scale is named after Scottish physicist and mathematician Lord Kelvin (1824-1907). It is based on molecular motion, with the temperature of 0 K, also known as absolute zero, being the point where all molecular motion ceases. The Kelvin scale was created by observing the relationship between pressure and temperature of low-density gases. Experiment shows that the pressure in a sealed volume of gas decreases as temperature decreases, and that a graph of pressure versus temperature is a straight line. For a given volume, both the slope of this line and the pressure at a given temperature depend on the quantity of gas in the volume. But no matter of what kind of gas or what quantity of gas is used, if we extrapolate the lines to low temperatures and pressures, we find that the pressure goes to zero at the same temperature. Each line should be extrapolated, because at sufficiently low temperatures, any gas becomes liquid.
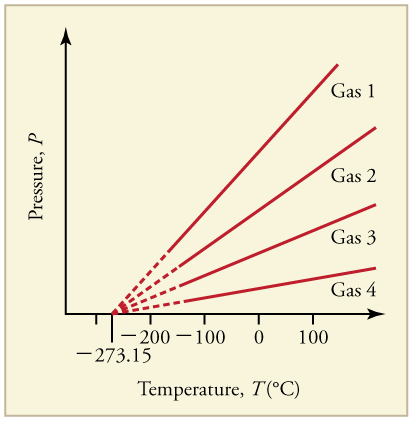
Source: http://cnx.org/contents/2ou0Jg2y@3/Temperature
This temperature is referred to as
absolute zero
, because lower temperatures are not physically possible, so temperatures on the Kelvin scale are
never
negative.
The unit of temperature used in the Kelvin scale is the Kelvin, abbreviated K. The value of the Kelvin is based on a reproducible temperature that can be precisely measured, which is the temperature of the
triple point
of water. The triple point of a substance is the pressure and temperature of that substance at which the solid, liquid, and vapor phases of the substance all simultaneously coexist. For water, the triple point pressure is 0.00603 atmospheres and the triple point temperature is defined to be 273.16 K. With this choice of the value of the Kelvin, a change of 1 ºC is exactly equal to a change of 1 K. The triple point of water turns out to be at 0.01 ºC, so 0 ºC corresponds to 273.15 K and 0 K (absolute zero) corresponds to – 273.15ºC. To convert from a temperature in Kelvin to degrees Celsius, simply subtract 273.15 and to convert a Celsius temperature to a Kelvin temperature just add 273.15.
Therefore, the conversion equation is: T(K) = T(ºC ) + 273.15
Water freezes at approximately 0 ºC = 273.15 K and boils at approximately 100 ºC = 373.15 K. It is good to remember that the precise values of these temperatures depend on the atmospheric pressure. It is very important to remember to use Kelvin when performing calculations described below that involve temperature.